Rational design of asymmetric benzodithiophene based photovoltaic polymers for efficient solar cells†
Abstract
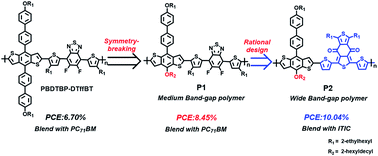
Extending π-conjugation in the benzodithiophene (BDT) side chains has been proven useful to improve the efficiencies of the BDT-based polymer solar cells (PSCs). Herein, combined with a symmetry-breaking strategy of a BDT unit, we further designed a new asymmetric 1D–2D (one dimensional–two dimensional) monomer asy-BDTBP with an alkoxyl group as the 1D part and a π-extending alkoxybiphenyl as the 2D substituted group. Medium band-gap donor–acceptor (D–A) conjugated polymer P1 was synthesized with asy-BDTBP and 4,7-di(4-(2-ethylhexyl)-2-thienyl)-5,6-difluoro-2,1,3-benzothiadiazole (DTffBT) as the donor and acceptor unit, respectively. Encouragingly, P1 blended with PC71BM exhibited an obviously enhanced power conversion efficiency (PCE) compared to the reported symmetric analogue PBDTBP–DTffBT (6.70%). The PCE increased to 8.45% with an open-circuit voltage (VOC) of 0.838 V, a short-circuit current density (JSC) of 14.35 mA cm−2 and a fill factor (FF) of 70.27%. However, P1 coupled with a classical non-fullerene acceptor ITIC revealed a relatively poor efficiency of 6.35% due to the bad complementarity of absorption spectra. To match the absorption of ITIC, a wide band-gap D–A polymer P2 was further designed with a weak electron-withdrawing group benzo[1,2-c:4,5-c′]dithiophene-4,8-dione (BDD) instead of DTffBT as the acceptor unit. As a result, P2 possessed a complementary absorption spectrum with ITIC, and the resulting devices presented an excellent photovoltaic performance. The optimal efficiency boosted to 10.04% with VOC of 0.873 V, JSC of 17.60 mA cm−2 and FF of 65.37%. This work demonstrates the great potential of asymmetric BDTs for high efficient PSCs and the importance of the rational design of polymers for different types of PSCs.


 Please wait while we load your content...
Please wait while we load your content...