Electrochemically substituted metal phthalocyanines, e-MPc (M = Co, Ni), as highly active and selective catalysts for CO2 reduction†
Abstract
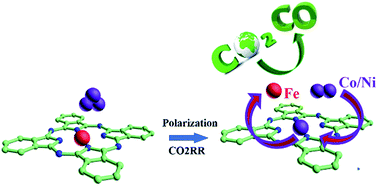
Metal-oxide nanoclusters (NCs) such as FeOx, CoOx, and NiOx are incorporated into iron phthalocyanine (FePc) supported on graphene, MOx/FePc, via a facile self-assembly method. MOx/FePc electrocatalysts show high activity, selectivity and stability for the electrochemical CO2 reduction reaction (CO2RR) as compared with the corresponding metal phthalocyanines, i.e., FePc, CoPc and NiPc, under identical test conditions. Near edge X-ray absorption fine structure (NEXAFS) spectroscopy reveals that MOx/FePc undergoes a metal ion replacement of the iron center of Pc, forming electrochemically substituted metal Pc, e-MPc where M = Co and Ni, co-existing with the replaced FeOx nanoparticles (NPs) in the vicinity of the e-MPc. The results indicate that the e-MPc with in situ dispersed FeOx NPs, FeOx/e-CoPc and FeOx/e-NiPc exhibits excellent activity, high selectivity and stability for the CO2RR.


 Please wait while we load your content...
Please wait while we load your content...