Polybenzimidazole membranes with nanophase-separated structure induced by non-ionic hydrophilic side chains for vanadium flow batteries†
Abstract
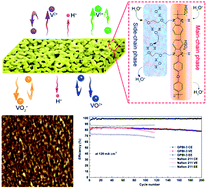
Polybenzimidazole (PBI) membranes with nanophase-separated structure induced by non-ionic hydrophilic side chain are designed and fabricated for vanadium flow batteries (VFBs). The designed PBI membranes are prepared by grafting non-ionic hydrophilic side chains via an N-substitution reaction. This molecular modification induces nanophase separation and formation of hydrophilic clusters, which act as effective proton transfer pathways, hence dramatically improving the proton conductivity. Meanwhile, the vanadium permeability is inappreciable due to the appropriate size of hydrophilic clusters and Donnan exclusion of protonated grafted-PBI membranes (GPBI). Free from ion exchange groups, GPBI membranes maintain the good chemical stability of the pristine PBI membrane. As a result, the designed membrane exhibits an impressive performance, combining ultrahigh proton conductivity, ion selectivity and chemical stability. The GPBI-based VFB exhibits a coulombic efficiency of over 99% and an energy efficiency of 84% at 120 mA cm−2, the highest reported for dense PBI membranes for VFB applications. The decent stability of GPBI membranes is demonstrated by the stable performance over 200 charge–discharge cycles and the ex situ immersion test. This work provides a new insight into the design of high-performance PBI membranes for VFB applications.


 Please wait while we load your content...
Please wait while we load your content...