Platinum loaded tin dioxide: a model system for unravelling the interplay between heterogeneous catalysis and gas sensing†
Abstract
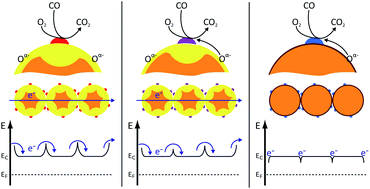
The presented work combines state-of-the-art material characterization with operando spectroscopy to unravel the complex structure–function-relationships which determine the gas sensing properties of Pt-loaded SnO2. It was found that platinum forms nanometer-sized oxide clusters at the tin dioxide surface, which act as the major reaction sites for CO oxidation strongly increasing the reactivity of the material. There is no direct correlation between the material's reactivity and gas sensing performance: oxidation catalysis requires an efficient CO oxidation and restoration of the initial state, while gas sensing requires reversible changes of the initial state, which can be translated into an electrical signal. The Pt content of the material and the atmospheric conditions determine whether CO oxidation or CO sensing dominate. In addition, this work demonstrates that for the Pt loaded samples the gas sensing properties are controlled by a Fermi-level control mechanism: the electronic coupling of the two oxides determines the charge transport in the sensing layer, which now depends on composition of the platinum phase.


 Please wait while we load your content...
Please wait while we load your content...