Bulk properties and transport mechanisms of a solid state antiperovskite Li-ion conductor Li3OCl: insights from first principles calculations†
Abstract
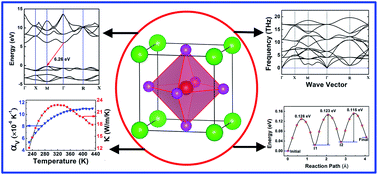
The excellent Li+ conductivity (0.85 × 10−3 S cm−1 at room temperature) and wide electrochemical stability window (>6 V) of the antiperovskite Li3OCl material make it a promising candidate electrolyte for rechargeable all-solid-state Li batteries. In this study, we systematically evaluate the electronic, mechanical, and thermodynamic properties of Li3OCl by first-principles density functional theory calculations. The defect chemistry and Li+ migration mechanisms are also discussed in the context since Li+ diffusion is strongly influenced by defects in the material. Our results show that Li3OCl is an indirect wide-band gap insulator in the equilibrium state with a band gap of ∼6.26 eV. Phonon spectral data confirm that Li3OCl is dynamically stable at its ground state. It is also revealed that Li3OCl is mechanically brittle. The bulk modulus of Li3OCl is greater than that of Li10GeP2S12, while it is comparable to that of Li0.5La0.5TiO3 and Li7La3Zr2O12. From quasi-harmonic approximation, the linear thermal expansion coefficient and thermal conductivity of the material are found to be 3.12 × 10−6 K−1 and 22.49 W m−1 K−1 at room temperature, respectively. With four types of point defect pairs in Li3OCl being considered, it is revealed that the LiCl defect pair has the lowest formation energy compared to Li2O, O substituted Cl, and Li/Li-vacancy Frenkel defect pairs. The LiCl and Frenkel defect pairs are the most important point defects responsible for the fast Li diffusion. Overall, our study provides fundamental and comprehensive insights into the bulk properties and transport mechanisms of Li3OCl for its practical application as a solid-state electrolyte in all-solid-state Li batteries.


 Please wait while we load your content...
Please wait while we load your content...