Scalable one-step electrochemical deposition of nanoporous amorphous S-doped NiFe2O4/Ni3Fe composite films as highly efficient electrocatalysts for oxygen evolution with ultrahigh stability†
Abstract
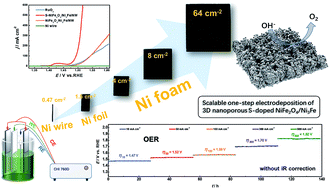
The rational design of noble metal-free electrocatalysts that are highly active, robustly stable, and capable of delivering large current densities (>500 mA cm−2) at low applied potentials (<300 mV), in particular for oxygen evolution reaction (OER), is critical for practical use in electro-driven water-splitting devices. Herein, we report a facile scalable and one-step electrochemical deposition approach for the development of a self-supported 3D nanoporous S-doped amorphous NiFe2O4/Ni3Fe composite electrode with outstanding OER electrocatalytic activity and robust durability in alkaline media. Benefiting from the 3D nanoporous architectures and their in situ growth on a highly conductive substrate, the novel S-doped NiFe2O4/Ni3Fe composite electrode offers an ideal platform for fast electron transport and efficient mass transport. It also provides abundant active surface area as well as accessible active sites for a catalytic reaction. Impressively, the S-doped composite electrode displays superior OER activity in 1.0 M KOH that compares favorably with the state-of-the-art RuO2 catalyst. Low overpotentials of 260 and 285 mV (iR corrected) are required to reach long-term stable current densities of 100 and 500 mA cm−2 for OER, respectively. The electrolyzer cell obtained by pairing this S-doped composite electrode as an anode with a Ni–Mo based cathode for overall water-splitting works efficiently in both 1.0 M (1.52 V for 10 mA cm−2 and 1.79 V for 100 mA cm−2) and 30 wt% KOH (1.69 V for 100 mA cm−2) solutions, with long durability for over 220 h. Such catalyst couple exhibits superior catalytic performance and holds great promise for potential application in electrochemical water splitting.


 Please wait while we load your content...
Please wait while we load your content...