High-rate oxygen electroreduction over metal-free graphene foams embedding P–N coupled moieties in acidic media†
Abstract
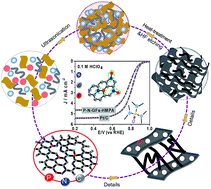
High-efficiency metal-free carbon electrocatalysts for the oxygen reduction reaction (ORR) in acidic medium are still a great challenge for the commercialization of the fuel cell technology. Herein, high-performance metal-free P,N-doped carbon catalysts (P–N-GFs-HMPA) with the E1/2 potential of about 0.78 V are successfully prepared by constructing porous foams using graphene oxides and carbon nanotubes as building bricks and hexamethylphosphoric triamide (HMPA) as the special precursor of P and N. Impressively, the HMPA compound not only facilitated the remarkable increase in the content of both P and N in the carbon frameworks, but also offered high density of the coupled N–P moieties embedded in the graphene surface. Together with the optimized local structures including open porosities and high electron transportation capacity, this foam exhibits excellent electrocatalytic performance for the ORR in acidic medium. It is markedly superior to the previously reported metal-free carbons and comparable to Fe- or Co-based ORR carbon electrocatalysts. Thus we have successfully developed a high-performance metal-free ORR catalyst in acidic medium by precisely tuning the carbon structure through coupling with non-metal elements.


 Please wait while we load your content...
Please wait while we load your content...