Crystal structure and surface characteristics of Sr-doped GdBaCo2O6−δ double perovskites: oxygen evolution reaction and conductivity†
Abstract
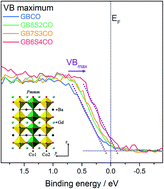
A cheap and direct solution towards engineering better catalysts through identification of novel materials is required for a sustainable energy system. Perovskite oxides have emerged as potential candidates to replace the less economically attractive Pt and IrO2 water splitting catalysts. In this work, excellent electrical conductivity (980 S cm−1) was found for the double perovskite of composition GdBa0.6Sr0.4Co2O6−δ which is consistent with a better oxygen evolution reaction activity with the onset polarisation of 1.51 V with respect to a reversible hydrogen electrode (RHE). GdBa1−xSrxCo2O6−δ with increasing Sr content was found to crystallise in the higher symmetry tetragonal P4/mmm space group in comparison with the undoped GdBaCo2O6−δ which is orthorhombic (Pmmm), and yields higher oxygen uptake, accompanied by higher Co oxidation states. This outstanding electrochemical performance is explained by the wider carrier bandwidth, which is a function of Co–O–Co buckling angles and Co–O bond lengths. Furthermore the higher oxygen evolution activity was observed despite the formation of non-lattice oxides (mainly hydroxide species) and enrichment of alkaline earth ions on the surface.
- This article is part of the themed collection: Advances in Solid State Chemistry and its Applications


 Please wait while we load your content...
Please wait while we load your content...