Catalytic and networking effects of carbon black on the kinetics and conversion of sulfur vulcanization in styrene butadiene rubber
Abstract
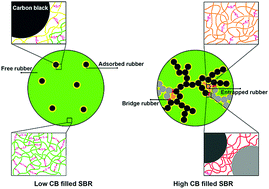
The present work discusses the effects of carbon-black (CB) on the kinetics and conversion of sulfur vulcanization in styrene butadiene rubber (SBR) compounds. Kinetic studies revealed that the onset of the vulcanization reaction shortens monotonically by incorporation of CB, but the rate of vulcanization goes through a maximum at a critical loading of CB. It was demonstrated that CB has two roles in the kinetics of vulcanization: a catalytic effect on accelerating the initial reactions among vulcanization agents and a networking effect on retarding the crosslinking of macro-radicals. It was shown that the latter effect dominates the former one at high concentrations of CB where the rubber-mediated network of CB is formed and a large portion of rubber is immobilized as bound rubber. By using two types of CBs with very different specific surface areas, it was discussed that the critical loading at which the retarding effect begins coincides with the rheological percolation threshold of CBs. Moreover, conversion of vulcanization under isothermal conditions was continuously reduced as the concentration of CBs increased. This was correlated to the magnitude of the physical restrictions exerted by CBs, depending on the specific surface area of each CB. However, it was also shown that this restriction could be alleviated at higher temperatures during non-isothermal vulcanization, which enhances the degree of conversion in crosslinking.


 Please wait while we load your content...
Please wait while we load your content...