Two one-dimensional arrays of naphthyl and anthryl groups along peptide nanotubes prepared from cyclic peptides comprising α- and β-amino acids†
Abstract
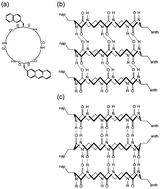
A novel cyclic hexapeptide composed of L-α-naphthylalanine, D-α-anthrylalanine, and four β-alanines (CP6) is synthesized and its molecular assembly into peptide nanotubes (PNTs) and the electronic properties arising from one-dimensional arrays of aromatic groups along the PNTs were investigated. CP6 with a combination of L- and D-α-amino acids is designed to self-assemble into PNTs with them stacking on top of each other under the constraint of maximizing the number of intermolecular hydrogen bonds between the cyclic peptides. Upon PNT formation, the respective side chains of L- and D-α-amino acids are aligned in line along the PNTs. The topological arrangement of the anthryl groups being in close proximity in the CP6 PNT is supported by higher photo-excited energy transfer, appearance of the induced Cotton effects, and the promoted photo-dimerization reaction upon PNT formation. AFM observations reveal that PNT bundles with diameters 5–15 nm are dielectric microcrystals having a piezoelectric coefficient of 2–6 pC N−1. Kelvin force microscopy observations show the generation of surface potentials over 100 mV owing to the one-dimensional array of the anthryl groups along PNTs. Incorporation of α-amino acids with opposite chirality into cyclic β-peptides is therefore an effective molecular design for the nano-architecture of PNTs displaying one-dimensional arrays of chromophores along PNTs.


 Please wait while we load your content...
Please wait while we load your content...