Charge and hydration structure of dendritic polyelectrolytes: molecular simulations of polyglycerol sulphate†
Abstract
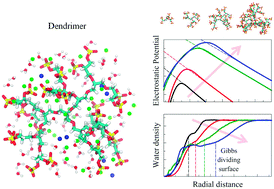
Macromolecules based on dendritic or hyperbranched polyelectrolytes have been emerging as high potential candidates for biomedical applications. Here we study the charge and solvation structure of dendritic polyglycerol sulphate (dPGS) of generations 0 to 3 in aqueous sodium chloride solution by explicit-solvent molecular dynamics computer simulations. We characterize dPGS by calculating several important properties such as relevant dPGS radii, molecular distributions, the solvent accessible surface area, and the partial molecular volume. In particular, as the dPGS exhibits high charge renormalization effects, we address the challenges of how to obtain a well-defined effective charge and surface potential of dPGS for practical applications. We compare implicit- and explicit-solvent approaches in our all-atom simulations with the coarse-grained simulations from our previous work. We find consistent values for the effective electrostatic size (i.e., the location of the effective charge of a Debye–Hückel sphere) within all the approaches, deviating at most by the size of a water molecule. Finally, the excess chemical potential of water insertion into dPGS and its thermodynamic signature are presented and rationalized.
- This article is part of the themed collection: Electrostatics and Soft Matter


 Please wait while we load your content...
Please wait while we load your content...