Specific ion effects for polyelectrolytes in aqueous and non-aqueous media: the importance of the ion solvation behavior†‡
Abstract
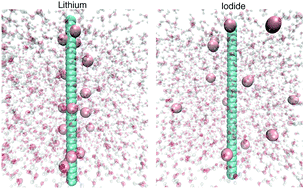
We present the results of atomistic molecular dynamics simulations with regard to specific ion effects in water, methanol and N,N-dimethylacetamide (DMAc). As a reference system, we introduce rigid and rod-like models of polyanions and polycations in combination with alkali metal cations and halide anions as counterions. Pronounced specific ion effects can be observed in terms of the individual anion and cation condensation behavior. The outcomes of our simulations thus reveal significant deviations from standard electrostatic mean-field theories. A detailed investigation of the individual energy contributions shows that ion–dipole interactions play a pivotal role in rationalizing the findings. The corresponding deviations in terms of the cation and anion distribution can be brought into agreement with the donor and acceptor numbers of the solvents, which thus highlights the importance of solvent–ion interactions in addition to electrostatic attraction.
- This article is part of the themed collection: Electrostatics and Soft Matter


 Please wait while we load your content...
Please wait while we load your content...