Ionic structure around polarizable metal nanoparticles in aqueous electrolytes
Abstract
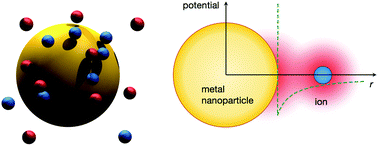
Metal nanoparticles are receiving increased scientific attention owing to their unique physical and chemical properties that make them suitable for a wide range of applications in diverse fields, such as electrochemistry, biochemistry, and nanomedicine. Their high metallic polarizability is a crucial determinant that defines their electrostatic character in various electrolyte solutions. Here, we introduce a continuum-based model of a metal nanoparticle with explicit polarizability in the presence of different kinds of electrolytes. We employ several, variously sophisticated, theoretical approaches, corroborated by Monte Carlo simulations in order to elucidate the basic electrostatics principles of the model. We investigate how different kinds of asymmetries between the ions result in non-trivial phenomena, such as charge separation and a build-up of a so-called zero surface-charge double layer.
- This article is part of the themed collection: Electrostatics and Soft Matter


 Please wait while we load your content...
Please wait while we load your content...