Emulsion stabilisation by complexes of oppositely charged synthetic polyelectrolytes†
Abstract
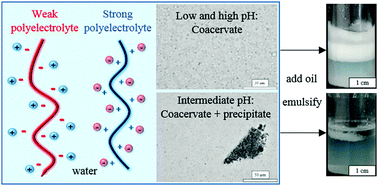
We investigate the possibility of stabilising oil–water emulsions from the polyelectrolyte complexes (PEC) obtained in mixtures of a strong cationic polyelectrolyte (poly(diallyldimethylammonium chloride), PDADMAC) and a weak anionic one (poly(acrylic acid)sodium salt, PAANa). Unlike other previous work however, both polyelectrolytes (PEL) are chosen as they are completely water-soluble and possess no surface activity when present alone over nearly all the pH range. In water, the effects of PEL concentration, PEL mixing ratio and pH on the formation of PEC are studied in detail. At low pH where the anionic PEL is uncharged, complex coacervation occurs in which droplets rich in both polymers are dispersed in water. At intermediate pH, the PEC comprise a mixture of coacervate droplets and solid particles. At high pH where the anionic PEL is significantly charged, only complex coacervation is observed. On addition of dodecane followed by homogenisation, no stable emulsions arose from dispersions containing solid particle PEC due to either the large precursor particle aggregates or their inherent hydrophilicity. By contrast, oil-in-water emulsions stable to coalescence could be prepared from coacervate dispersions. We discuss the feasibility of the coacervate phase spreading at the oil–water interface in terms of the relevant spreading coefficients and compare the predictions with experiment for a range of oils. We encounter oils whose drops become engulfed by the coacervate phase as well as oils where no engulfing occurs.


 Please wait while we load your content...
Please wait while we load your content...