Zirconia incorporated calcium looping absorbents with superior sintering resistance for carbon dioxide capture from in situ or ex situ processes†
Abstract
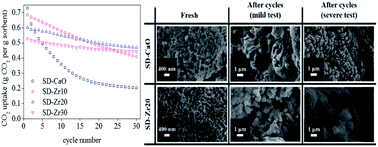
The sintering deactivation of Ca-based absorbents has greatly limited the application of calcium looping for either in situ or ex situ processes. In this work, a series of CaO sorbents stabilized with CaZrO3 were prepared by three different wet chemistry methods: freeze drying, spray drying and heating drying. Multi-cycle CO2 capture tests under relatively mild conditions demonstrated that the spray-dried sorbents performed best. Based on both total CO2 uptake and the deactivation rate, the optimum content of CaZrO3 was 20 wt%. Under severe conditions, the best sorbent could even retain a capacity of 0.47 g and 0.44 g CO2 per g sorbent at cycle 30 and cycle 100, respectively, which showed notable superiority over previously reported similar materials. The stabilization mechanism was explored by characterization. It was found that the introduction of CaZrO3 increased the specific surface area and the spray drying method generated a spherical structure, which could retain some pores after severe tests. Three semi-empirical equations were introduced to describe the evolution of the decay process.


 Please wait while we load your content...
Please wait while we load your content...