Electrocatalytic reduction of CO2 to produce higher alcohols†
Abstract
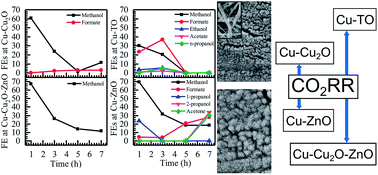
Electrodeposited and thermally oxidized copper surfaces have been documented in recent years to produce simple alcohols. In this work, we endeavored to study the electrochemical reduction of CO2 at different electrodes prepared via the electrodeposition method, namely, Cu–Cu2O, Cu–Cu2O–ZnO, and Cu–ZnO. In addition, thermally oxidized Cu (Cu-TO) was also investigated. C1, C2, and C3 species were produced on Cu–Cu2O–ZnO, Cu–Cu2O, and Cu–ZnO. The highest faradaic efficiency (FE of 97.4%) of the liquid products (methanol, formate, n-propanol, acetone) was evidenced on Cu–ZnO. The formation of C3 species with high FE on the Cu–ZnO electrode is attributed to the fast C–C–C coupling at the Cu–Zn interface. On thermally oxidized Cu, the total FE of the liquid products (methanol, formate, ethanol, acetate, n-propanol) was found to be 58.51%, which is considerably closer to the already reported values for these electrodes. Moreover, the Cu–Cu2O–ZnO electrode revealed selectivity toward methanol production. Detailed morphological and elemental analyses of the electrode, performed using XPS, Raman spectroscopy, and FESEM, as well as activity measurements to obtain an insight into the mechanistic pathways, reveal that C–C coupling is favored on Cu0 sites rather than Cu2O. Moreover, methanol formation seems to proceed via O coordination of CO2 to Cu–Cu2O surface having (100) facets, whereas C coordination is favored on Cu-TO with (111) exposed faces, resulting in Cu0 sites. The localized formation of ZnO nanoflowers was observed on Cu–ZnO electrodes after the electrochemical reduction of CO2, which is attributed to the mechanistic pathway involving chemical steps, leading to the formation of C3 species.


 Please wait while we load your content...
Please wait while we load your content...