Electrochemical membrane cell for NH3 synthesis from N2 and H2O by electrolysis at 200 to 250 °C using a Ru catalyst, hydrogen-permeable Pd membrane and phosphate-based electrolyte
Abstract
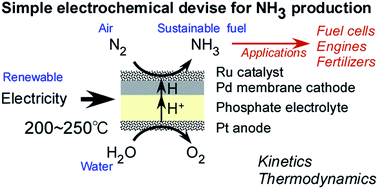
Ammonia (NH3) is an energy carrier that can be synthesized from nitrogen and water using electricity generated from renewable sources. The present work investigated NH3 synthesis using an electrochemical system with the structure of Ru/Cs+/MgO|Pd–Ag|CsH2PO4/SiP2O7|Pt and operating at 200 to 250 °C. In this system, the NH3 being generated is isolated from the CsH2PO4/SiP2O7 electrolyte by the hydrogen-permeable Pd–Ag membrane, resulting in the production of dry NH3. A maximum NH3 synthesis rate of 0.90 nmol s−1 cm−2 was obtained from the cathode at the Ru/Cs+/MgO|Pd–Ag side at a current density of 10 mA cm−2, temperature of 250 °C and N2 flow rate of 3 cm3 min−1 as converted to standard temperature and pressure. Deviations in the NH3 production rate from the theoretical amounts predicted by Arrhenius plots were observed at temperatures approaching 250 °C, possibly because the reaction approached equilibrium. A current efficiency for NH3 production of 2.6% was obtained, with the remainder of the current consumed during H2 generation. The apparent activation energies for the NH3 synthesis were 69 and 93 kJ mol−1 at 3.2 and 10 mA cm−2, respectively. These values are significantly lower than that reported for conventional NH3 synthesis from nitrogen and hydrogen (139 kJ mol−1) over the same catalyst in this cell. The optimum N2 flow rates were approximately 0.5 and 5 cm3 min−1 at 3.2 and 10 mA cm−2, respectively, representing nitrogen amounts approximately 42 times the stoichiometric quantities. The provision of an excess of N2 is believed to reduce the suppression of N2 activation by so-called hydrogen poisoning of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...