Synthesis of yolk–shell spheres based on molybdenum diselenide-encapsulated molybdenum oxide for efficient electrocatalytic hydrogen evolution†
Abstract
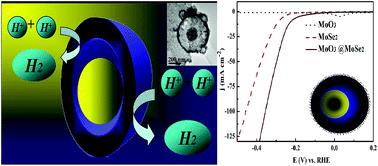
Transition metal dichalcogenides for electrocatalytic water splitting are important in renewable energy research, with studies showing that the edge structure and specific surface area are crucial to their electrocatalytic activity. Herein, the design and construction of yolk–shell nanospheres based on molybdenum diselenide-encapsulated molybdenum oxide are reported. Their structures were characterized using various techniques, which showed that the void space between the interior core and outer shell increased the number of active sites. This yolk–shell structure exhibited enhanced and stable electrocatalytic activity in the hydrogen evolution reaction (HER). The HER current density at −0.5 V vs. the reversible hydrogen electrode was increased by 95% (to −256 mA cm−2) compared to that of traditional flower-like molybdenum diselenide. The overpotential of this yolk–shell structure, which was required to drive a current density of −10 mA cm−2, was 65 mV lower than that of flower-like molybdenum diselenide (286 mV). Furthermore, long-term potential cycling and potentiostatic measurement of this catalyst showed favorable stability and durability of electrocatalytic activity. This yolk–shell structure plays a key role in energy conversion processes central to several renewable energy technologies.


 Please wait while we load your content...
Please wait while we load your content...