A cheap metal for a challenging task: nickel-catalyzed highly diastereo- and enantioselective hydrogenation of tetrasubstituted fluorinated enamides†
Abstract
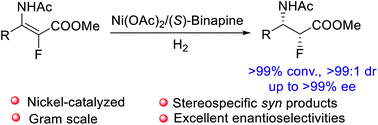
Nickel-catalyzed asymmetric hydrogenation of challenging tetrasubstituted fluorinated enamides has been achieved, affording chiral α-fluoro-β-amino esters in high yields with excellent diastereo- and enantioselectivities (up to 98% yield, >99 : 1 dr, up to >99% ee). Deuterium-labeling experiments and control experiments were conducted to probe the mechanism, and the results indicated that the acidity of the solvent plays a critical role in the control of diastereoselectivity by trapping the adduct of nickel hydride to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds via protonolysis, giving the hydrogenation product with stereospecific syn-selectivity. This protocol provides efficient access to chiral α-fluoro-β-amino esters which have important potential applications in organic synthesis and medicinal chemistry.
C bonds via protonolysis, giving the hydrogenation product with stereospecific syn-selectivity. This protocol provides efficient access to chiral α-fluoro-β-amino esters which have important potential applications in organic synthesis and medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...