Site-selective protein conjugation at histidine†
Abstract
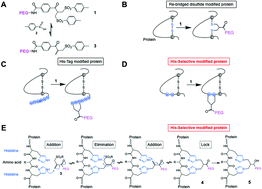
Site-selective conjugation generally requires both (i) molecular engineering of the protein of interest to introduce a conjugation site at a defined location and (ii) a site-specific conjugation technology. Three N-terminal interferon α2-a (IFN) variants with truncated histidine tags were prepared and conjugation was examined using a bis-alkylation reagent, PEG(10kDa)-mono-sulfone 3. A histidine tag comprised of two histidines separated by a glycine (His2-tag) underwent PEGylation. Two more IFN variants were then prepared with the His2-tag engineered at different locations in IFN. Another IFN variant was prepared with the His-tag introduced in an α-helix, and required three contiguous histidines to ensure that two histidine residues in the correct conformation would be available for conjugation. Since histidine is a natural amino acid, routine methods of site-directed mutagenesis were used to generate the IFN variants from E. coli in soluble form at titres comparable to native IFN. PEGylation conversions ranged from 28–39%. A single step purification process gave essentially the pure PEG–IFN variant (>97% by RP-HPLC) in high recovery with isolated yields ranging from 21–33%. The level of retained bioactivity was strongly dependent on the site of PEG conjugation. The highest biological activity of 74% was retained for the PEG10-106(HGHG)-IFN variant which is unprecedented for a PEGylated IFN. The His2-tag at 106(HGHG)-IFN is engineered at the flexible loop most distant from IFN interaction with its dimeric receptor. The biological activity for the PEG10-5(HGH)-IFN variant was determined to be 17% which is comparable to other PEGylated IFN conjugates achieved at or near the N-terminus that have been previously described. The lowest retained activity (10%) was reported for PEG10-120(HHH)-IFN which was prepared as a negative control targeting a IFN site thought to be involved in receptor binding. The presence of two histidines as a His2-tag to generate a site-selective target for bis-alkylating PEGylation is a feasible approach for achieving site-selective PEGylation. The use of a His2-tag to strategically engineer a conjugation site in a protein location can result in maximising the retention of the biological activity following protein modification.
- This article is part of the themed collections: Most popular 2019-2020 chemical biology articles and 2019 International Open Access Week Collection


 Please wait while we load your content...
Please wait while we load your content...