Redox-triggered cascade dearomative cyclizations enabled by hexafluoroisopropanol†
Abstract
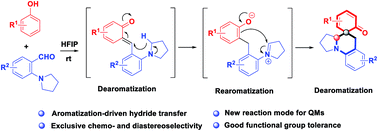
An unprecedented cascade dearomative cyclization through hydrogen-bonding-assisted hydride transfer is realized. The aggregate effect of HFIP enables the rapid buildup of polycyclic amines directly from phenols and o-aminobenzaldehydes via a cascade dearomatization/rearomatization/dearomatization sequence. This unique transformation addressed the drawbacks of hydride transfer-involved redox-neutral reactions.


 Please wait while we load your content...
Please wait while we load your content...