Direct observation of the intermediate in an ultrafast isomerization†
Abstract
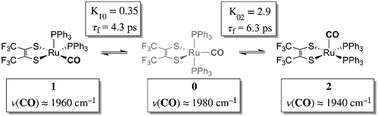
Using a combination of two-dimensional infrared (2D IR) and variable temperature Fourier transform infrared (FTIR) spectroscopies the rapid structural isomerization of a five-coordinate ruthenium complex is investigated. In methylene chloride, three exchanging isomers were observed: (1) square pyramidal equatorial, (1); (2) trigonal bipyramidal, (0); and (3) square pyramidal apical, (2). Exchange between 1 and 0 was found to be an endergonic process (ΔH = 0.84 (0.08) kcal mol−1, ΔS = 0.6 (0.4) eu) with an isomerization time constant of 4.3 (1.5) picoseconds (ps, 10−12 s). Exchange between 0 and 2 however was found to be exergonic (ΔH = −2.18 (0.06) kcal mol−1, ΔS = −5.3 (0.3) eu) and rate limiting with an isomerization time constant of 6.3 (1.6) ps. The trigonal bipyramidal complex was found to be an intermediate, with an activation barrier of 2.2 (0.2) kcal mol−1 and 2.4 (0.2) kcal mol−1 relative to the equatorial and apical square pyramidal isomers respectively. This study provides direct validation of the mechanism of Berry pseudorotation – the pairwise exchange of ligands in a five-coordinate complex – a process that was first described over fifty years ago. This study also clearly demonstrates that the rate of pseudorotation approaches the frequency of molecular vibrations.
- This article is part of the themed collections: 2019 Chemical Science HOT Article Collection and Editor's Choice – Serena DeBeer


 Please wait while we load your content...
Please wait while we load your content...