Insight into wild-type and T1372E TET2-mediated 5hmC oxidation using ab initio QM/MM calculations†
Abstract
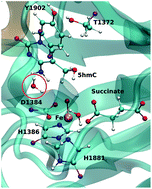
Ten-eleven translocation 2 (TET2) is an Fe/α-ketoglutarate (α-KG) dependent enzyme that dealkylates 5-methylcytosine (5mC). The reaction mechanism involves a series of three sequential oxidations that convert 5mC to 5-hydroxy-methylcytosine (5hmC), 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC). Our previous biochemical and computational studies uncovered an active site scaffold that is required for wild-type (WT) stepwise oxidation (Nat. Chem. Bio., 13, 181). We showed that the mutation of a single residue, T1372 to some amino acids, such as Glu, can impact the iterative oxidation steps and stop the oxidation of 5hmC to 5fC/caC. However, the source of the stalling at the first oxidation step by some mutant TET proteins still remains unclear. Here, we studied the catalytic mechanism of oxidation of 5hmC to 5fC by WT and T1372E TET2 using an ab initio quantum mechanical/molecular mechanical (QM/MM) approach. Our results suggest that the rate limiting step for WT TET2 involves a hydrogen atom abstraction from the hydroxyl group of 5hmC by the ferryl moiety in the WT. By contrast, our calculations for the T1372E mutant indicate that the rate limiting step for this variant corresponds to a second proton abstraction and the calculated barrier is almost twice as large as for WT TET2. Our results suggest that the large barrier for the 5hmC to 5fC oxidation in this mutant is due (at least in part) to the unfavorable orientation of the substrate in the active site. Combined electron localization function (ELF) and non-covalent interaction (NCI) analyses provide a qualitative description of the evolution of the electronic structure of the active site along the reaction path. Energy decomposition analysis (EDA) has been performed on the WT to investigate the impact of each MM residue on catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...