meta-C–H arylation of fluoroarenes via traceless directing group relay strategy†
Abstract
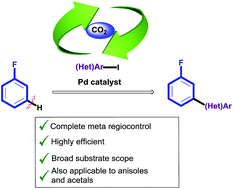
While several methods for the ortho selective arylation of fluoroarenes, meta-functionalisation has never been achieved. We report a new methodology, based on the traceless directing group relay concept, leading to the first meta-selective (hetero)arylation of fluoroarenes. In this strategy, CO2 is introduced as a transient directing group, to control a Pd-catalysed arylation meta to the fluoro functionality, prior to its release in a sequential, one-pot fashion. This method has shown compatibility with a number of functional groups and substitution patterns in both the fluoroarene core and aryl iodide coupling partners, and proceeds with complete meta-selectivity and mono vs. bis-arylation selectivity.
- This article is part of the themed collections: Most popular 2018-2019 catalysis articles and Celebrating our 2019 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...