Hydrogen-bonded perylene bisimide J-aggregate aqua material†
Abstract
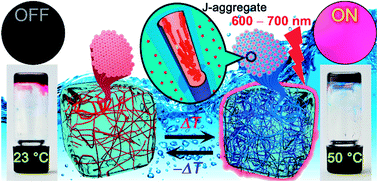
A new twelvefold methoxy-triethyleneglycol-jacketed tetraphenoxy-perylene bisimide (MEG-PBI) amphiphile was synthesized that self-assembles into two types of supramolecular aggregates in water: red-coloured aggregates of low order and with weak exciton coupling among the PBIs and blue-coloured strongly coupled J-aggregates consisting of a highly ordered hydrogen-bonded triple helix of PBIs. At room temperature this PBI is miscible with water at any proportions which enables the development of robust dye aggregates in solution, in hydrogel states and in lyotropic liquid crystalline states. In the presence of 60–95 wt% water, self-standing coloured hydrogels exhibit colour changes from red to blue accompanied by a fluorescence light-up in the far-red region upon heating in the range of 30–50 °C. This phenomenon is triggered by an entropically driven temperature-induced hydrogen-bond-directed slipped stacking arrangement of the MEG-PBI chromophores within structurally well-defined J-aggregates. This versatile aqua material is the first example of a stable PBI J-aggregate in water. We anticipate that this study will open a new avenue for the development of biocompatible functional materials based on self-assembled dyes and inspire the construction of other hydrogen-bonded supramolecular materials in the highly competitive solvent water.
- This article is part of the themed collection: Most popular 2018-2019 supramolecular chemistry articles


 Please wait while we load your content...
Please wait while we load your content...