Synthesis of aryl-thioglycopeptides through chemoselective Pd-mediated conjugation†
Abstract
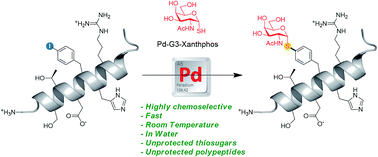
We describe herein a Pd-catalyzed methodology for the thioglycoconjugation of iodoaryl peptides and aminoacids. This operationally simple process occurs under semi-aqueous conditions and displays wide substrate scope. The strategy has been successfully applied to both the thioglycosylation of unprotected peptides and the generation of thioglyco-aminoacid building blocks, including those suitable for solid phase peptide synthesis. To demonstrate the broad potential of this technique for late stage functionalization, we successfully incorporated challenging unprotected β-S-GlcNAc- and α-S-GalNAc-derivatives into very long unprotected peptides. This study opens the way to new applications in chemical biology, considering the well-recognized advantages of S-glycosides over O-glycosides in terms of resistance towards both enzymatic and chemical degradation.


 Please wait while we load your content...
Please wait while we load your content...