Understanding the visible-light photocatalytic activity of GaN:ZnO solid solution: the role of Rh2−yCryO3 cocatalyst and charge carrier lifetimes over tens of seconds†‡
Abstract
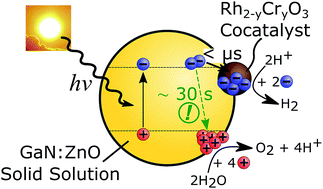
A persistent challenge for the widespread deployment of solar fuels is the development of high efficiency photocatalysts combined with a low-cost preparation and implementation route. Since its discovery in 2005, GaN:ZnO solid solution has been a benchmark overall water splitting photocatalyst. Notably, GaN:ZnO functionalised with an appropriate proton reduction cocatalyst is one of the few particulate photocatalyst systems that can generate hydrogen and oxygen directly from water using visible light. However, the reasons underlying the remarkable visible light activity of GaN:ZnO are not well understood and photophysical studies of GaN:ZnO have been limited to date. Using time-resolved optical spectroscopies, we investigated the charge carrier dynamics of GaN:ZnO and the effect of Rh2−yCryO3 proton reduction cocatalyst. Here we show that charge trapping and trap state filling play an important role in controlling the photophysics of GaN:ZnO. We also find that electrons transfer to Rh2−yCryO3 on sub-microsecond timescales, important to reduce the electron concentration within GaN:ZnO and promote hole accumulation. Operando measurements showed that the water oxidation process is the rate determining process, and that the dependence of the rate of water oxidation on the accumulated hole density is similar to common metal oxides photoanodes such as TiO2, α-Fe2O3, and BiVO4. Remarkably, we show that the recombination timescale of holes accumulated on the surface of GaN:ZnO is on the order of 30 s, distinctly longer than for metal oxides photoanodes. We conclude that the unusual visible light activity of GaN:ZnO is a result of large electron–hole spatial separation due to the preferential flow of holes to the GaN-rich surface and efficient electron extraction by the cocatalyst. Our studies demonstrate that in depth spectroscopic investigations of the charge carrier dynamics of photocatalysts yield important information to understand their behaviour, and identify key properties to deliver outstanding performance.
- This article is part of the themed collection: In celebration of Kazunari Domen’s 65th birthday, 2018


 Please wait while we load your content...
Please wait while we load your content...