Design and synthesis of a 4-aminoquinoline-based molecular tweezer that recognizes protoporphyrin IX and iron(iii) protoporphyrin IX and its application as a supramolecular photosensitizer†
Abstract
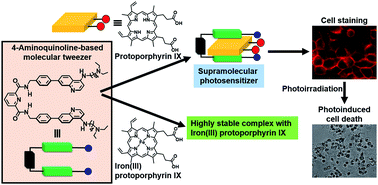
We report on the design and synthesis of a new type of 4-aminoquinoline-based molecular tweezer 1 which forms a stable host–guest complex with protoporphyrin IX (PPIX) via multiple interactions in a DMSO and HEPES buffer (pH 7.4) mixed solvent system. The binding constant for the 1 : 1 complex (K11) between 1 and PPIX is determined to be 4 × 106 M−1. Furthermore, 1 also forms a more stable complex with iron(III) protoporphyrin IX (Fe(III)PPIX), the K11 value for which is one order of magnitude greater than that for PPIX, indicating that 1 could be used as a recognition unit of a synthetic heme sensor. On the other hand, the formation of the stable PPIX·1 complex (supramolecular photosensitizer) prompted us to apply it to photodynamic therapy (PDT). Cell staining experiments using the supramolecular photosensitizer and evaluations of its photocytotoxicity indicate that the PDT activity of PPIX is improved as the result of the formation of a complex with 1.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...