Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol†
Abstract
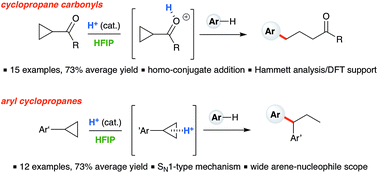
Ring-opening hydroarylation of cyclopropanes is typically limited to substrates bearing a donor–acceptor motif. Here, the transformation is achieved for monosubstituted cyclopropanes by using catalytic Brønsted acid in hexafluoroisopropanol (HFIP) solvent, constituting a rare example where such cyclopropanes engage in intermolecular C–C bond formation. Branched products are obtained when electron-rich arylcyclopropanes react with a broad scope of arene nucleophiles in accord with a simple SN1-type ring-opening mechanism. In contrast, linear products are obtained when cyclopropylketones react with electron-rich arene nucleophiles. In the latter case, mechanistic experiments and DFT-calculations support a homo-conjugate addition pathway.
- This article is part of the themed collection: The ChemRxiv Collection


 Please wait while we load your content...
Please wait while we load your content...