Highly diastereoselective synthesis of tricyclic fused-pyrans by sequential hydride shift mediated double C(sp3)–H bond functionalization†
Abstract
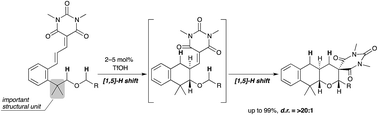
Described herein is the Brønsted acid-catalyzed double C(sp3)–H bond functionalization of alkyl phenethyl ether derivatives. In this process, a [1,5]-[1,5]-hydride shift occurred successively to afford tricyclic fused pyran derivatives in excellent chemical yields with excellent diastereoselectivities (up to >20 : 1). The key to achieve this reaction was the introduction of two methyl groups at the benzylic position, which was effective in both hydride shift processes: (1) the Thorpe–Ingold effect for the first hydride shift and (2) conformational control in the second hydride shift.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...