New Ru(ii) photocages operative with near-IR light: new platform for drug delivery in the PDT window†
Abstract
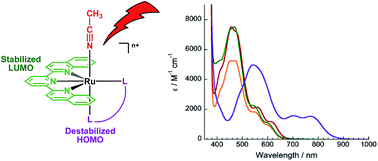
A series of Ru(II) complexes bearing the tridentate 2,6-di(quinolin-2-yl)pyridine (dqpy) ligand were designed to undergo photoinduced ligand dissociation with red/near-IR light. The complexes [Ru(dqpy)(L)(CH3CN)]2+, where L = 2,2′-bipyridine (bpy, 1), 4,4′dimethyl-2,2′-bipyridine (Me2bpy, 2), and 1,10-phenanthroline (phen, 3). Complexes 1–3 exhibit red-shifted lowest energy metal-to-ligand charge transfer (MLCT) absorption maxima at ∼600 nm, as compared to the corresponding tpy (2,2′;6′,2′′-terpyridine) complexes with MLCT bands at ∼565 nm which appear as shoulders to the MLCT bands at ∼455 nm. This shift is attributed to the lower energy LUMO afforded by the dqpy ligand when compared to tpy, as evidenced by the shift of the first reduction wave to ∼0.3 V more positive potentials in the former. In addition, the lowest MLCT maximum of [Ru(dqpy)(acac)(CH3CN)]+ (4; acac− = acetylacetonate) is observed at 770 nm, attributed to the additional increase in energy of the HOMO afforded by the presence of the π-donating acac− ligand and supported by calculations. Complexes 1–3 undergo ligand substitution upon irradiation with red light, λirr ≥ 610 nm, and the ligand substitution photochemistry of 4 is accessible with near-IR light, λirr ≥ 715 nm and λirr = 735 nm. Complexes 1–4 exhibit similar quantum yields of ligand exchange, ΦL, with 450 and 600 nm irradiation, however, that of 4 is 2–3 times greater than those measured for 1–3. This enhancement is explained by the difference in ligand contributions to the HOMO. Density functional theory calculations predict partial dqpy ππ* character in the MLCT states of 1–3 and a mixed Ru/acac− → dqpy metal/ligand-to-ligand charge transfer (ML-LCT) state in 4. The photoreactivity of 1–4 with tissue-penetrating red and near-IR light, together with their exceptional dark stability (>48 h), makes the new Ru(II)-dqpy platform ideal for the development of new complexes for photoinduced drug release and for other applications that require broad absorption from the ultraviolet and visible ranges into the near-IR, such as solar energy conversion.


 Please wait while we load your content...
Please wait while we load your content...