Reversibly tuning hydrogel stiffness through photocontrolled dynamic covalent crosslinks†
Abstract
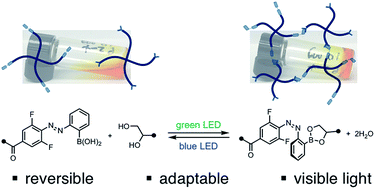
Controlling the physical properties of soft materials with external stimuli enables researchers to mimic and study dynamic systems. Of particular interest are hydrogels, polymer networks swollen by water with broad applicability to biomedicine. To control hydrogel mechanics with light, researchers have relied on a limited number of photochemical reactions. Here we introduce an approach to reversibly tune hydrogel mechanics with light by manipulating the stability of dynamic covalent crosslinks at the molecular level. The equilibrium between a boronic acid and diol to form a boronic ester can be altered by the configuration of an adjacent azobenzene photoswitch. By irradiating branched polymers bearing azobenzene-boronic acid and diol end groups with two different wavelengths of light, we can stiffen or soften the resulting hydrogel. Alternating irradiation induces reversible mechanical changes. Rheological characterization reveals that the hydrogels are viscoelastic, exhibiting stress relaxation on the order of seconds, and the stiffness is tuned independently of the crossover frequency. We have also demonstrated that this approach can be extended to use visible light for both softening and stiffening. These photocontrolled dynamic covalent crosslinks provide a versatile platform for tunable dynamic materials.
- This article is part of the themed collections: New reactivity in organic chemistry and Editors’ Choice Collection


 Please wait while we load your content...
Please wait while we load your content...