Diastereo- and enantioselective copper catalyzed hydroallylation of disubstituted cyclopropenes†
Abstract
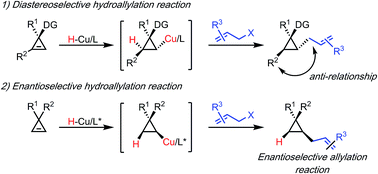
A highly diastereo- and enantioselective protocol for the hydroallylation of 1,1- and 1,2-disubstituted cyclopropenes has been developed utilizing an in situ formed copper hydride. A variety of allyl electrophiles could be utilized yielding a diverse range of trisubstituted cyclopropanes. Finally a preliminary enantioselective variant could be established employing a recently described P-stereogenic xantphos derivative as ligand.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...