A snapshot of the electrochemical reaction layer by using 3 dimensionally resolved fluorescence mapping†
Abstract
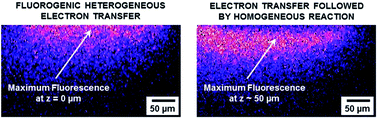
The coupling between electrochemistry and fluorescence confocal laser scanning microscopy (FCLSM) allows deciphering the electrochemical and/or redox reactivity of electroactive fluorophores. This is demonstrated with phenoxazine electrofluorogenic species frequently used in bioassays by mapping the variation of fluorescence intensity with respect to the distance from the electrode. The electrochemical conversion of resorufin dye (RF) to non-fluorescent dihydroresorufin (DH) leads to a sharp decrease of the fluorescence signal in the vicinity of the electrode. In contrast, the direct reduction of resazurin (RZ) to DH leads to an unexpected maximum fluorescence intensity localized further away from the surface. This observation indicates that the initial electron transfer (heterogeneous) is followed by a chemical comproportionation step (homogeneous), leading to the formation of RF within the diffusion layer with a characteristic concentration profile. Therefore, in situ FCLSM affords a direct way to monitor such chemical reactivity in space and to decipher a new redox pathway that cannot be resolved solely by electrochemical means.


 Please wait while we load your content...
Please wait while we load your content...