Hydrogen evolution from water catalyzed by cobalt-mimochrome VI*a, a synthetic mini-protein†
Abstract
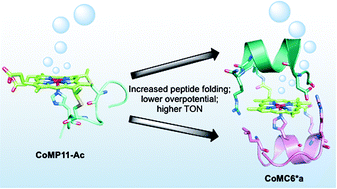
A synthetic enzyme is reported that electrocatalytically reduces protons to hydrogen (H2) in water near neutral pH under aerobic conditions. Cobalt mimochrome VI*a (CoMC6*a) is a mini-protein with a cobalt deuteroporphyrin active site within a scaffold of two synthetic peptides covalently bound to the porphyrin. Comparison of the activity of CoMC6*a to that of cobalt microperoxidase-11 (CoMP11-Ac), a cobalt porphyrin catalyst with a single “proximal” peptide and no organized secondary structure, reveals that CoMC6*a has significantly enhanced longevity, yielding a turnover number exceeding 230 000, in comparison to 25 000 for CoMP11-Ac. Furthermore, comparison of cyclic voltammograms of CoMC6*a and CoMP11-Ac indicates that the trifluoroethanol-induced folding of CoMC6*a lowers the overpotential for catalytic H2 evolution by up to 100 mV. These results demonstrate that even a minimal polypeptide matrix can enhance longevity and efficiency of a H2-evolution catalyst.


 Please wait while we load your content...
Please wait while we load your content...