Chiral Brønsted acid-catalyzed intramolecular SN2′ reaction for enantioselective construction of a quaternary stereogenic center†
Abstract
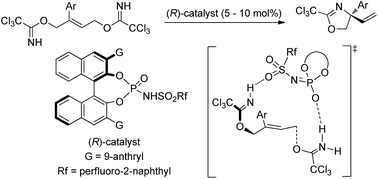
An enantioselective intramolecular anti-SN2′ cyclization reaction for the construction of a quaternary stereogenic center was accomplished through the activation of the leaving group using a binaphthol-derived phosphoramide as the chiral Brønsted acid catalyst. The present allylic substitution reaction is beneficial not only for the regioselective nucleophilic substitution at the multi-substituted site of the double bond but also for controlling the stereochemical outcome because of using a geometrically defined double bond. Indeed, the reaction afforded synthetically useful amino alcohol derivatives having a tetra-substituted carbon center in a highly enantioselective manner in most cases, in which the modification of the sulfonamide unit of the phosphoramide catalyst was demonstrated to improve the enantioselectivity. Experimental and theoretical elucidation of the reaction mechanism suggested that the reaction proceeds through a synchronous anti-SN2′ pathway, although NMR monitoring of the reaction indicated the formation of the phosphorimidate ester via the SN2 reaction of the catalyst with the substrate, which results in catalyst deactivation. Further theoretical studies of the origin of the stereochemical outcome at the generated quaternary stereogenic center were performed. Structural analysis of the transition states at the enantio-determining step revealed that the distinct discrimination of the substituents attached to the geometrically defined double bond is achieved by the anthryl and sulfonamide substituents of the catalyst through the three-point hydrogen bonding interactions and the T-shaped C–H⋯π interactions.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...