Concentrated electrolytes stabilize bismuth–potassium batteries†
Abstract
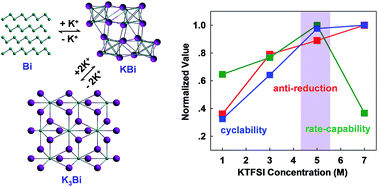
Storing as many as three K-ions per atom, bismuth is a promising anode material for rechargeable potassium-ion batteries that may replace lithium-ion batteries for large-scale electrical energy storage. However, Bi suffers from poor electrochemical cyclability in conventional electrolytes. Here, we demonstrate that a 5 molar (M) ether-based electrolyte, versus the typical 1 M electrolyte, can effectively passivate the bismuth surface due to elevated reduction resistance. This protection allows a bismuth–carbon anode to simultaneously achieve high specific capacity, electrochemical cyclability and Coulombic efficiency, as well as small potential hysteresis and improved rate capability. We show that at a high electrolyte concentration, the bismuth anode demonstrates excellent cyclability over 600 cycles with 85% capacity retention and an average Coulombic efficiency of 99.35% at 200 mA g−1. This “concentrated electrolyte” approach provides unexpected new insights to guide the development of long-cycle-life and high-safety potassium-ion batteries.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...