Fine-tuned organic photoredox catalysts for fragmentation-alkynylation cascades of cyclic oxime ethers†
Abstract
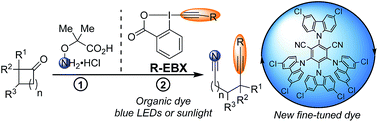
Fine-tuned organic photoredox catalysts are introduced for the metal-free alkynylation of alkylnitrile radicals generated via oxidative ring opening of cyclic alkylketone oxime ethers. The redox properties of the dyes were determined by both cyclic voltammetry and computation and covered an existing gap in the oxidation potential of photoredox organocatalysts.
- This article is part of the themed collections: Most popular 2018-2019 catalysis articles, Editor's choice - Paolo Melchiorre, Functional Organic Materials Symposium Collection and 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...