Synthesis of cyclic chiral α-amino boronates by copper-catalyzed asymmetric dearomative borylation of indoles†
Abstract
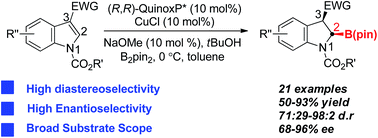
A copper(I)-catalyzed dearomative borylation of N-alkoxycarbonyl protected indole-3-carboxylates has been developed. The boron addition in this reaction occurred regioselectively at the 2-position of indoles followed by diastereoselective protonation, affording the corresponding stable cyclic chiral α-amino boronates (2-borylindolines) in moderate to good yields with excellent diastereo- and enantioselectivities. The product 2c could be used as a versatile precursor to undergo subsequent stereoselective transformations, delivering highly functionalized 2,3,3-trisubstituted chiral indolines.


 Please wait while we load your content...
Please wait while we load your content...