Direct evidence for heme-assisted solid-state electronic conduction in multi-heme c-type cytochromes†
Abstract
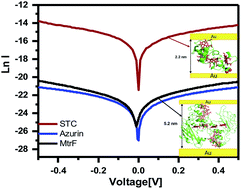
Multi-heme cytochrome c (Cytc) proteins are key for transferring electrons out of cells, to enable intracellular oxidation to proceed in the absence of O2. In these proteins most of the hemes are arranged in a linear array suggesting a facile path for electronic conduction. To test this, we studied solvent-free electron transport across two multi-heme Cytc-type proteins: MtrF (deca-heme Cytc) and STC (tetra-heme Cytc). Transport is measured across monolayers of these proteins in a solid state configuration between Au electrodes. Both proteins showed 1000× higher conductance than single heme, or heme-free proteins, but similar conductance to monolayers of conjugated organics. Conductance is found to be temperature-independent (320–80 K), suggesting tunneling as the transport mechanism. This mechanism is consistent with I–V curves modelling, results of which could be interpreted by having protein-electrode coupling as rate limiting, rather than transport within the proteins.


 Please wait while we load your content...
Please wait while we load your content...