Exploring a new ligand binding site of G protein-coupled receptors†
Abstract
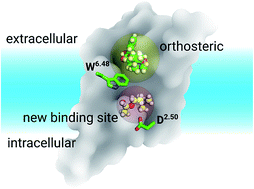
Identifying a target ligand binding site is an important step for structure-based rational drug design as shown here for G protein-coupled receptors (GPCRs), which are among the most popular drug targets. We applied long-time scale molecular dynamics simulations, coupled with mutagenesis studies, to two prototypical GPCRs, the M3 and M4 muscarinic acetylcholine receptors. Our results indicate that unlike synthetic antagonists, which bind to the classic orthosteric site, the endogenous agonist acetylcholine is able to diffuse into a much deeper binding pocket. We also discovered that the most recently resolved crystal structure of the LTB4 receptor comprised a bound inverse agonist, which extended its benzamidine moiety to the same binding pocket discovered in this work. Analysis on all resolved GPCR crystal structures indicated that this new pocket could exist in most receptors. Our findings provide new opportunities for GPCR drug discovery.


 Please wait while we load your content...
Please wait while we load your content...