Unusual biaryl torsional strain promotes reactivity in Cu-catalyzed Sommelet–Hauser rearrangement†
Abstract
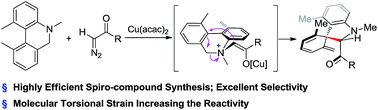
A Cu-catalyzed Sommelet–Hauser dearomatization of dihydrophenanthridine and diazo compounds is reported for the synthesis of spiro-indolines. A spiro-structure with adjacent quaternary and tertiary carbon centers was constructed in one step as a single isomer. Increasing steric hindrance by introducing ortho-substituents dramatically improved substrate reactivity in this transformation.


 Please wait while we load your content...
Please wait while we load your content...