Pyrene hydrogel for promoting direct bioelectrochemistry: ATP-independent electroenzymatic reduction of N2†
Abstract
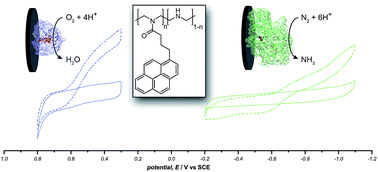
Enzymatic bioelectrocatalysis often requires an artificial redox mediator to observe significant electron transfer rates. The use of such mediators can add a substantial overpotential and obfuscate the protein's native kinetics, which limits the voltage of a biofuel cell and alters the analytical performance of biosensors. Herein, we describe a material for facilitating direct electrochemical communication with redox proteins based on a novel pyrene-modified linear poly(ethyleneimine). This method was applied for promoting direct bioelectrocatalytic reduction of O2 by laccase and, by immobilizing the catalytic subunit of nitrogenase (MoFe protein), to demonstrate the ATP-independent direct electroenzymatic reduction of N2 to NH3.
- This article is part of the themed collections: Biohybrid approaches for energy conversion, Functional Organic Materials Symposium Collection, 2018 Chemical Science HOT Article Collection, Celebrating Excellence in Research: 100 Women of Chemistry, Editors’ Choice Collection and Collection to celebrate our diverse and global authorship


 Please wait while we load your content...
Please wait while we load your content...