Deciphering the mechanism of O2 reduction with electronically tunable non-heme iron enzyme model complexes†
Abstract
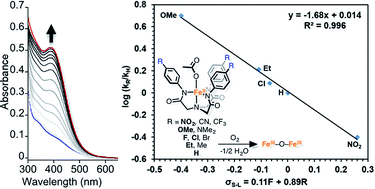
A homologous series of electronically tuned 2,2′,2′′-nitrilotris(N-arylacetamide) pre-ligands (H3LR) were prepared (R = NO2, CN, CF3, F, Cl, Br, Et, Me, H, OMe, NMe2) and some of their corresponding Fe and Zn species synthesized. The iron complexes react rapidly with O2, the final products of which are diferric mu-oxo bridged species. The crystal structure of the oxidized product obtained from DMA solutions contain a structural motif found in some diiron proteins. The mechanism of iron mediated O2 reduction was explored to the extent that allowed us to construct an empirically consistent rate law. A Hammett plot was constructed that enabled insightful information into the rate-determining step and hence allows for a differentiation between two kinetically equivalent O2 reduction mechanisms.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...