Enantioselective radical process for synthesis of chiral indolines by metalloradical alkylation of diverse C(sp3)–H bonds†
Abstract
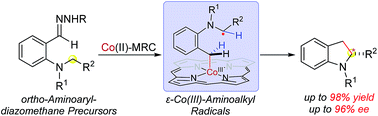
A new C–C bond formation strategy based on enantioselective radical alkylation of C(sp3)–H bonds via Co(II)-based metalloradical catalysis has been demonstrated for stereoselective synthesis of chiral indolines. The Co(II)-based system enables activation of aryldiazomethanes as radical precursors at room temperature for enantioselective intramolecular radical alkylation of broad types of C–H bonds, constructing 2-substituted indolines in high yields with excellent enantioselectivities. In addition to chemoselectivity and regioselectivity, this Co(II)-catalyzed alkylation features tolerance to functional groups and compatibility with heteroaryl substrates. Detailed mechanistic studies provide insight into the underlying stepwise radical pathway.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...