A supramolecular radical cation: folding-enhanced electrostatic effect for promoting radical-mediated oxidation†
Abstract
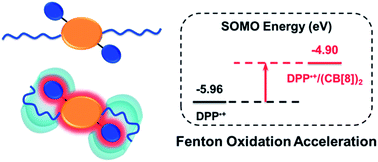
We report a supramolecular strategy to promote radical-mediated Fenton oxidation by the rational design of a folded host–guest complex based on cucurbit[8]uril (CB[8]). In the supramolecular complex between CB[8] and a derivative of 1,4-diketopyrrolo[3,4-c]pyrrole (DPP), the carbonyl groups of CB[8] and the DPP moiety are brought together through the formation of a folded conformation. In this way, the electrostatic effect of the carbonyl groups of CB[8] is fully applied to highly improve the reactivity of the DPP radical cation, which is the key intermediate of Fenton oxidation. As a result, the Fenton oxidation is extraordinarily accelerated by over 100 times. It is anticipated that this strategy could be applied to other radical reactions and enrich the field of supramolecular radical chemistry in radical polymerization, photocatalysis, and organic radical battery and holds potential in supramolecular catalysis and biocatalysis.


 Please wait while we load your content...
Please wait while we load your content...