Revitalizing the concept of bond order through delocalization measures in real space†
Abstract
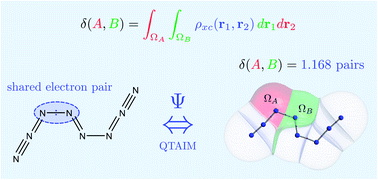
Ab initio quantum chemistry is an independent source of information supplying an ever widening group of experimental chemists. However, bridging the gap between these ab initio data and chemical insight remains a challenge. In particular, there is a need for a bond order index that characterizes novel bonding patterns in a reliable manner, while recovering the familiar effects occurring in well-known bonds. In this article, through a large body of calculations, we show how the delocalization index derived from Quantum Chemical Topology (QCT) serves as such a bond order. This index is defined in a parameter-free, intuitive and consistent manner, and with little qualitative dependency on the level of theory used. The delocalization index is also able to detect the subtler bonding effects that underpin most practical organic and inorganic chemistry. We explore and connect the properties of this index and open the door for its extensive usage in the understanding and discovery of novel chemistry.
- This article is part of the themed collections: Most popular 2018-2019 physical and theoretical chemistry articles, The ChemRxiv Collection, 2018 ChemSci Pick of the Week Collection and 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...