Pd(ii)-catalyzed synthesis of bifunctionalized carboranes via cage B–H activation of 1-CH2NH2-o-carboranes†
Abstract
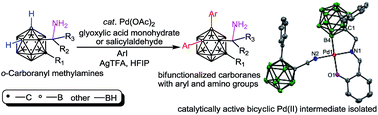
Aminoalkyl carboranes are anticipated to be valuable synthons toward the synthesis of bifunctionalized carboranes. However, direct cage boron derivation of these carborane derivatives has not been solved. Herein, the reversible conversion of catalytically infeasible o-carboranyl methylamines (1-CH2NH2-o-carboranes) into bidentate imines initiates Pd-mediated cage B–H activation. As a result, an amine coordinated bicyclic Pd(II) complex (3) has been isolated and proven to be the catalytically active intermediate for highly site-selective B–H diarylation of o-carboranyl methylamines. Using glyoxylic acid as an inexpensive and commercially available transient directing reagent, a wide range of cage B(4,5)-diarylated free primary o-carboranyl methylamines were prepared in good to excellent yields with the avoidance of the pre-installation and removal of a directing group. This method provides easy access to cage boron functionalized o-carboranyl methylamines with potential for application in pharmaceuticals.
- This article is part of the themed collection: Most popular 2018-2019 main group, inorganic and organometallic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...