Modular access to functionalized 5–8–5 fused ring systems via a photoinduced cycloisomerization reaction†
Abstract
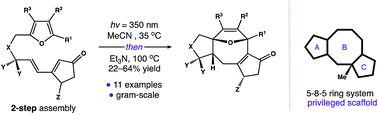
A 5–8–5 carbocyclic ring system forms the core of over 30 distinct natural products. Several members of this family have gained attention for their diverse activity in cell culture. In these cases, biological function is mediated by the arrangement of substituents around a conserved 5–8–5 nucleus. Despite the potential applications of this privileged substructure in medicinal chemistry, modular strategies for its assembly are underdeveloped. Herein, we describe a cycloisomerization reaction that forms the 5–8–5 framework directly. This strategy uniquely allows access to gram quantities of this valuable scaffold in four steps.


 Please wait while we load your content...
Please wait while we load your content...