Gold-catalyzed (4 + 2)-annulations between α-alkyl alkenylgold carbenes and benzisoxazoles with reactive alkyl groups†
Abstract
This work reports new (4 + 2)-annulations of α-alkyl vinylgold carbenes with benzisoxazoles to afford 3,4-dihydroquinoline derivatives with high anti-stereoselectivity. The annulations are operable with carbenes in both acyclic and cyclic forms. This reaction sequence involves an initial formation of imines from α-alkylgold carbenes and benzisoxazoles, followed by a novel carbonyl-enamine reaction to yield 3,4-dihydroquinoline derivatives. This system presents the first alkyl C–H reactivity of α-alkyl gold carbenes with an external substrate.
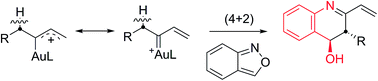
- This article is part of the themed collection: Most popular 2018-2019 catalysis articles


 Please wait while we load your content...
Please wait while we load your content...